avogadro’s number questions and answers pdf
Avogadro’s Number is a fundamental constant in chemistry‚ linking moles to the number of particles. It’s essential for calculations involving gases‚ molar masses‚ and reaction stoichiometry.
What is Avogadro’s Number?
Avogadro’s Number is a fundamental constant in chemistry‚ defined as 6.02214076 × 10²³ particles per mole. It serves as a conversion factor between macroscopic and microscopic quantities‚ enabling calculations involving gases‚ solutions‚ and chemical reactions. This number represents the amount of atoms‚ molecules‚ or particles in one mole of a substance. For example‚ one mole of carbon atoms contains approximately 6.022 × 10²³ atoms. Avogadro’s Number is essential in stoichiometry‚ molar mass calculations‚ and understanding gas laws. Its precise value ensures accuracy in scientific computations‚ making it a cornerstone of modern chemistry. Without it‚ converting between grams‚ moles‚ and particles would be impossible‚ hindering advancements in fields like materials science and pharmacology.
Importance of Avogadro’s Number in Chemistry
Avogadro’s Number is foundational in chemistry‚ enabling precise calculations and conversions between macroscopic quantities (like grams) and microscopic particles (atoms‚ molecules). It is essential for stoichiometry‚ determining molar masses‚ and understanding gas behavior. By linking moles to particles‚ it facilitates calculations of concentrations‚ reaction yields‚ and molecular interactions. Its applications span chemistry‚ physics‚ and engineering‚ making it indispensable for scientists and researchers. Without Avogadro’s Number‚ modern chemistry would lack the ability to quantify matter at atomic and molecular scales‚ hindering advancements in fields like drug development and materials science. Its significance lies in its universality‚ providing a consistent bridge between laboratory measurements and theoretical models.

History and Significance
Avogadro’s Number‚ discovered by Amedeo Avogadro in 1811‚ is a cornerstone of modern chemistry‚ enabling precise calculations in stoichiometry and gas laws‚ with universal applications in science and engineering.
Who Discovered Avogadro’s Number?
Amedeo Avogadro‚ an Italian scientist‚ introduced the concept of Avogadro’s Number in 1811 through his hypothesis about gas particles. However‚ the exact value was not determined during his lifetime. In 1909‚ French physicist Jean Perrin experimentally determined the constant‚ now known as Avogadro’s Number‚ earning him the Nobel Prize in Physics in 1926. Avogadro’s work laid the foundation for modern chemistry‚ particularly in understanding molecular theory and stoichiometry. His discovery revolutionized how scientists quantify matter at the microscopic level‚ making it a cornerstone in chemical calculations and gas laws. Today‚ Avogadro’s Number is a fundamental constant‚ symbolizing the bridge between macroscopic and microscopic chemistry. Its significance extends beyond chemistry‚ influencing physics and engineering as well. Avogadro’s legacy continues to inspire advancements in science‚ highlighting the importance of his pioneering work.
Historical Development of Avogadro’s Number
The development of Avogadro’s Number began with Amedeo Avogadro’s 1811 hypothesis that equal volumes of gases contain equal numbers of particles. This idea‚ known as Avogadro’s Law‚ was initially met with skepticism but gained acceptance over time. In the late 19th century‚ scientists like Josef Loschmidt and Ludwig Boltzmann advanced the concept‚ leading to the estimation of the number of particles in one mole. The term “Avogadro’s Number” was coined in 1909 by Jean Perrin‚ who experimentally determined its value through Brownian motion studies. The number was initially approximated as 6.02 x 10²³ particles per mole. Over the years‚ precise measurements have refined this value‚ culminating in its modern definition as 6.02214076 x 10²³‚ a cornerstone in chemistry and physics. This evolution underscores the collaborative nature of scientific progress.

The Value and Applications
Avogadro’s Number is approximately 6.022 x 10²³ particles per mole‚ enabling precise calculations in chemistry‚ such as converting between grams‚ moles‚ and molecules‚ and is widely applied in stoichiometry and gas laws.
The Exact Value of Avogadro’s Number
The exact value of Avogadro’s Number is defined as 6.02214076 × 10²³ particles per mole‚ a constant in metrology and chemistry. This precise value was adopted in 2019 as part of the redefinition of the International System of Units (SI)‚ making it a foundational constant in scientific calculations. Avogadro’s Number is used to convert between the amount of a substance in moles and the number of particles (atoms‚ molecules‚ or ions). It is crucial in fields such as gas laws‚ stoichiometry‚ and molar mass calculations. For practical purposes‚ students often use an approximate value of 6.022 × 10²³ for simplicity. This constant is essential for understanding the relationship between macroscopic and microscopic quantities in chemistry‚ making it a cornerstone of chemical education and research.
Practical Applications of Avogadro’s Number
Avogadro’s Number has numerous practical applications in chemistry and related fields. It is used to calculate the number of particles (atoms‚ molecules‚ or ions) in a given amount of a substance. For instance‚ in stoichiometry‚ it helps determine the amount of reactants and products in chemical reactions. Gas laws rely heavily on Avogadro’s Number to relate volume‚ moles‚ and pressure. Additionally‚ it is essential in calculating molar masses and converting grams to moles. In laboratory settings‚ it aids in preparing precise solutions and understanding molecular structures. Avogadro’s Number is also used in real-world scenarios‚ such as in pharmacy to determine drug dosages and in environmental science to measure pollutant concentrations. Its applications are vast‚ making it a fundamental tool in scientific and industrial processes.

Calculations Using Avogadro’s Number
Avogadro’s Number is crucial for converting moles to particles‚ calculating molar masses‚ and solving stoichiometry problems. It simplifies understanding chemical reactions and gas laws‚ enabling precise calculations.
Basic Concepts of Moles and Molecules
Moles and molecules are foundational concepts in chemistry‚ enabling the quantification of substances. A mole represents 6.022 x 10²³ particles‚ aligning with Avogadro’s Number. Molecules are clusters of atoms bonded together‚ and understanding their structure is vital for chemical calculations. In problems involving moles‚ Avogadro’s Number acts as a conversion factor‚ bridging macroscopic and microscopic scales. For instance‚ calculating the number of molecules in a given moles of a substance is a common application. Worksheets and practice exams often include questions where students convert grams to moles using molar mass and then apply Avogadro’s Number to find the number of particles. Mastering these concepts is essential for solving stoichiometry problems and understanding chemical reactions.
Calculating the Number of Particles
Calculating the number of particles involves using Avogadro’s Number to convert moles to individual atoms‚ molecules‚ or formula units. This process is central to stoichiometry and chemical calculations. For example‚ to find the number of molecules in a given number of moles‚ multiply the moles by Avogadro’s Number (6.022 x 10²³ particles/mol). If the substance is provided in grams‚ first convert grams to moles using molar mass before applying Avogadro’s Number. Worksheets often include problems like calculating the number of atoms in a sample or determining the mass corresponding to a specific number of particles. Ensuring unit consistency is crucial‚ as errors in unit conversion can lead to incorrect results. Practice problems help students master these calculations‚ which are fundamental to understanding chemical reactions and quantitative analysis.
Converting Between Moles and Particles
Converting between moles and particles is a foundational skill in chemistry‚ utilizing Avogadro’s Number. This process involves multiplying or dividing by 6.022 x 10²³ particles/mol. To convert moles to particles‚ multiply the number of moles by Avogadro’s Number; For example‚ 2.5 moles of CO₂ molecules become 2.5 mol x 6.022 x 10²³ molecules/mol = 1.505 x 10²⁴ molecules. Conversely‚ to convert particles to moles‚ divide by Avogadro’s Number. For instance‚ 3.01 x 10²⁴ atoms of Fe divided by 6.022 x 10²³ atoms/mol equals approximately 0.5 mol of Fe. Worksheets and practice problems often include such conversions‚ ensuring accuracy and unit consistency. These exercises are vital for understanding chemical quantities and reactions‚ making them a cornerstone of chemistry education and problem-solving.
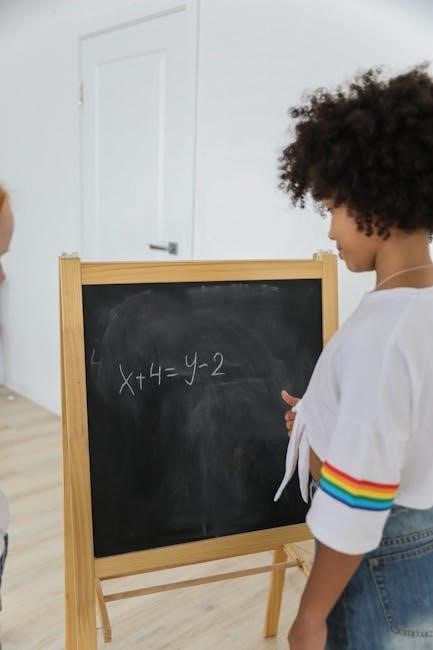
Avogadro’s Law and Gas Laws
Avogadro’s Law states that the volume of a gas is directly proportional to the number of moles‚ at constant temperature and pressure. This law is fundamental to understanding gas behavior and is often combined with other gas laws‚ such as Boyle’s and Charles’s laws‚ to solve real-world problems in chemistry.
Understanding Avogadro’s Law
Avogadro’s Law states that the volume of a gas is directly proportional to the number of moles of gas‚ provided temperature and pressure remain constant. This means that doubling the number of moles doubles the volume‚ assuming conditions stay unchanged. The law is expressed mathematically as V ∝ n or V1/n1 = V2/n2 when comparing two gas samples. It is a foundational principle in gas behavior and is widely used in stoichiometry and gas-related calculations. Avogadro’s Law helps explain how gases expand and contract under different conditions and is essential for understanding reactions involving gases. Worksheets and practice problems often use this law to teach students how to relate volume and moles in chemical systems. Its significance lies in its simplicity and applicability to real-world scenarios‚ making it a cornerstone of chemistry education.
Relating Volume and Moles
Avogadro’s Law establishes a direct relationship between the volume of a gas and the number of moles of gas particles. At constant temperature and pressure‚ doubling the number of moles doubles the volume. This relationship is mathematically expressed as V1/n1 = V2/n2‚ where V is volume and n is moles. This principle is crucial for solving problems involving gas reactions and stoichiometry. For example‚ if 1 mole of helium occupies 22.4 liters at STP‚ 2 moles would occupy 44.8 liters. Worksheets often include questions where students calculate volumes based on moles or vice versa‚ using Avogadro’s Number for conversions. Understanding this relationship is vital for predicting gas behavior and balancing chemical equations involving gases. Such problems are fundamental in chemistry education‚ helping students grasp the quantitative nature of gas laws and their practical applications in laboratory settings and real-world scenarios.
Gas Laws and Their Significance
The gas laws‚ including Boyle’s‚ Charles’s‚ and Avogadro’s‚ describe how gases respond to changes in pressure‚ volume‚ and temperature. These laws are foundational in chemistry and physics‚ enabling predictions of gas behavior under various conditions. Boyle’s Law relates pressure and volume‚ while Charles’s Law connects volume and temperature. Avogadro’s Law ties volume to the number of moles‚ emphasizing the role of Avogadro’s Number in gas calculations. Together‚ these laws form the ideal gas law‚ PV = nRT‚ which is widely used in scientific and engineering applications. Understanding gas laws is crucial for fields like aerospace engineering‚ industrial processes‚ and environmental science. They also highlight the importance of Avogadro’s Number in connecting macroscopic and microscopic properties of gases‚ making them essential for solving problems in stoichiometry and thermodynamics. Their significance extends to real-world applications‚ such as respiratory medicine and HVAC systems‚ showcasing their practical relevance.
Molar Mass and Conversions
Molar mass is the mass of one mole of a substance‚ calculated using atomic masses from the periodic table. It bridges grams to moles‚ enabling conversions using Avogadro’s Number.
What is Molar Mass?
Molar mass is the mass of one mole of a substance‚ measured in grams per mole (g/mol). It is calculated by summing the atomic masses of all atoms in a molecule‚ using the periodic table. For elements‚ the molar mass is the atomic mass of the element. For compounds‚ it is the sum of the molar masses of all constituent elements. For example‚ water (H₂O) has a molar mass of 18.015 g/mol: 2(1.008 for hydrogen) + 16.00 for oxygen. Molar mass is crucial for converting between grams and moles‚ enabling stoichiometric calculations in chemistry. It serves as a bridge between macroscopic and microscopic quantities‚ often used with Avogadro’s Number to determine particle counts.
Calculating Molar Mass
Molar mass is calculated by summing the atomic masses of all atoms in a compound‚ using the periodic table. For elements‚ it is simply the atomic mass. For compounds‚ it involves adding the molar masses of each element‚ multiplied by the number of atoms present. For example‚ water (H₂O) has a molar mass of 18.015 g/mol: 2(1.008 for hydrogen) + 16.00 for oxygen. For sodium chloride (NaCl)‚ it is 58.44 g/mol: 22.99 (Na) + 35.45 (Cl). Accurate calculations require using the atomic masses from the periodic table. Molar mass is a critical concept in chemistry‚ enabling conversions between grams and moles. It is often used with Avogadro’s Number to determine particle counts and solve stoichiometric problems. Always ensure units are consistent for accurate results.
Converting Grams to Moles
Converting grams to moles involves using the molar mass of a substance. Divide the given mass in grams by the molar mass (g/mol) to find the number of moles. For example‚ to convert 50 grams of NaCl to moles: 50 g ÷ 58.44 g/mol ≈ 0.855 moles. This conversion is essential in stoichiometry and chemical reactions. Ensure the units are consistent‚ and the molar mass is accurate. This method allows chemists to relate macroscopic quantities to microscopic particles using Avogadro’s Number. Always verify calculations for accuracy.

Practical Problems and Solutions
Practical problems often involve calculating moles‚ particles‚ and masses. Solutions require applying Avogadro’s Number and molar masses accurately. Common mistakes include unit errors and miscalculations‚ which can be avoided with careful step-by-step approaches. Always verify your work to ensure accuracy in your answers.
Sample Problems Involving Avogadro’s Number
Sample problems involving Avogadro’s Number often focus on mole-to-particle conversions and molar mass calculations. For example‚ determining the number of molecules in a given number of moles or calculating the mass of a substance based on its molar mass. One common problem asks: “How many molecules are in 5.00 moles of HCN?” Using Avogadro’s Number (6.02 x 10²³ particles/mol)‚ the solution involves multiplying the number of moles by the constant. Another example: “A chemist has 388.2 g of iron. How many moles does it contain?” This requires finding the molar mass of iron (55.85 g/mol) and dividing the given mass by it. These problems test understanding of stoichiometric relationships and unit conversions‚ ensuring accurate and precise calculations in chemistry.
Step-by-Step Solutions
Solving problems involving Avogadro’s Number requires a systematic approach. Start by identifying the given information and the unknown quantity. For example‚ to find the number of molecules in a given number of moles‚ multiply the moles by Avogadro’s Number (6.02 x 10²³ particles/mol). When calculating molar mass‚ sum the atomic masses of all atoms in the compound. For conversions‚ use dimensional analysis to ensure units cancel appropriately. Always show intermediate steps‚ such as converting grams to moles using molar mass before applying Avogadro’s Number. Verify the reasonableness of answers by checking unit consistency. Common mistakes include incorrect use of molar mass or forgetting to square units in calculations. Practicing with sample problems enhances understanding of mole-particle relationships and stoichiometry.
Common Mistakes to Avoid
When working with Avogadro’s Number‚ common errors include using the wrong value for the constant. Always use 6.02 x 10²³ particles/mol for calculations. Forgetting to convert grams to moles before applying Avogadro’s Number is another frequent mistake. Ensure to use molar mass for this conversion. Miscounting atoms in molecules‚ such as in CH₄ (5 atoms per molecule)‚ can lead to incorrect results. Confusing moles of substances with moles of particles is also a pitfall. For gases‚ remember that one mole equals 6.02 x 10²³ molecules or atoms. Mixing up units‚ like using grams instead of kilograms‚ can cause errors. Always check unit consistency and the reasonableness of answers. Practicing with sample problems and reviewing fundamental concepts helps minimize these errors and improves problem-solving skills.

Real-World Applications
Avogadro’s Number is crucial in real-world chemistry‚ from calculating drug dosages to determining the number of particles in industrial processes‚ ensuring accurate and efficient outcomes in various fields.
Everyday Examples Using Avogadro’s Number
Avogadro’s Number is often encountered in everyday scenarios‚ making it a practical tool for understanding the microscopic world. For instance‚ in cooking‚ it helps determine the number of molecules in ingredients‚ ensuring precise measurements. In medicine‚ pharmacists use it to calculate drug dosages‚ ensuring the correct number of molecules for effectiveness. Environmental scientists apply it to measure air quality by counting pollutant molecules. Even in hobbies like homebrewing‚ it aids in balancing chemical reactions for fermentation. These examples highlight how Avogadro’s Number bridges the gap between abstract chemistry and real-world applications‚ making it an indispensable concept in both professional and everyday contexts.
Avogadro’s Number in Laboratory Settings
Laboratories rely heavily on Avogadro’s Number for precise measurements and calculations. Scientists use it to determine the number of particles in a sample‚ ensuring accurate experimental results. In stoichiometric calculations‚ it helps quantify reactants and products‚ essential for chemical synthesis. Additionally‚ Avogadro’s Number is crucial in gas law applications‚ where the volume and pressure of gases are analyzed. Researchers also employ it in molar mass determinations and concentration calculations for solutions. Its role in laboratory settings underscores its importance in maintaining the precision and reproducibility of scientific experiments‚ making it an indispensable tool for chemists and researchers worldwide.










Leave a Comment